
A Simple Way To Get Atomic Mass Of First 20 Elements Of The Periodic Free Hot Nude Porn Pic
Get essential facts about the first 20 elements, all in one convenient place, including the name, atomic number, atomic mass, element symbol, group, and electron configuration. If you need detailed facts about these elements or any of the higher numbered ones, start with the clickable periodic table . 01 of 20 Hydrogen davidf / Getty Images

Periodic Table Elements Valency Charges Elements เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 1465162751
This table of element valences includes the maximum valence and most common valence values. Use this is a reference with a periodic table.

Valency of the elements from 1 to 30 Science Materials Metals and NonMetals 13851567
The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined - 4s 2, 4p 1). The completely filled d orbitals count as core, not valence, electrons. Transition elements or.
The first 20 Elements YouTube
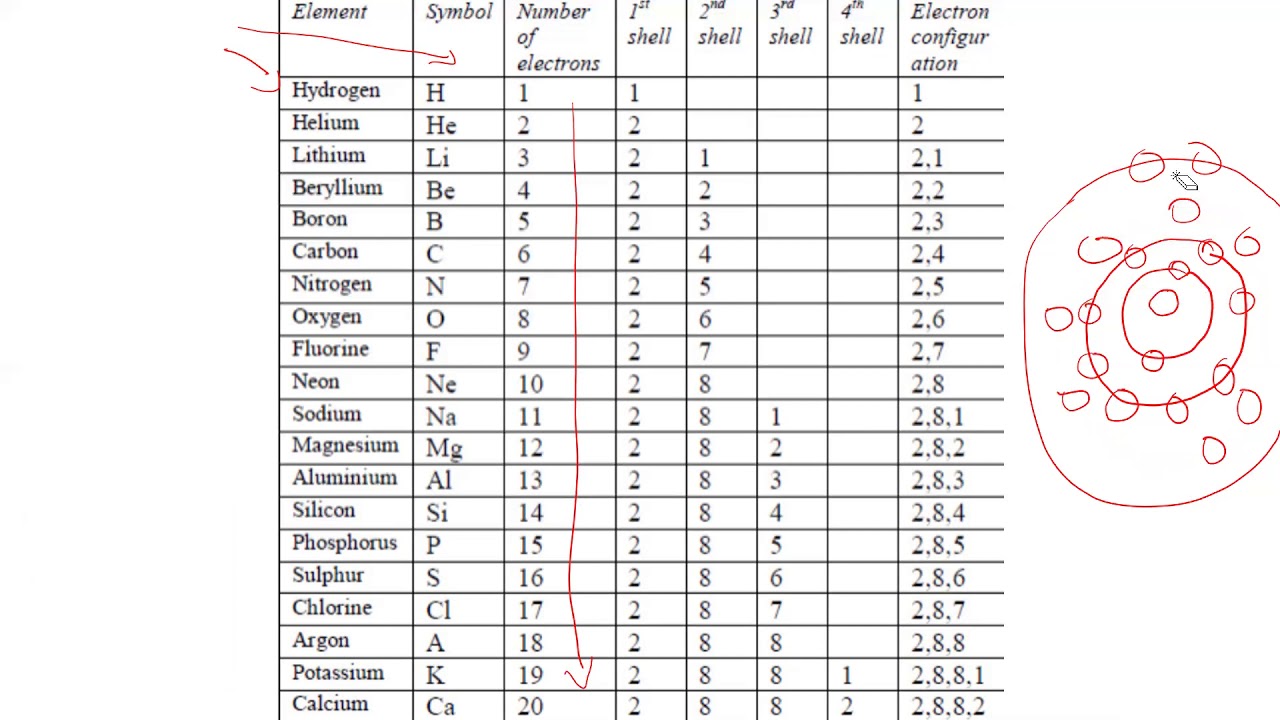
These are the first 20 elements, listed in order: H - Hydrogen He - Helium Li - Lithium Be - Beryllium B - Boron C - Carbon N - Nitrogen O - Oxygen F - Fluorine Ne - Neon Na - Sodium Mg - Magnesium Al - Aluminum Si - Silicon P - Phosphorus S - Sulfur Cl - Chlorine Ar - Argon K - Potassium Ca - Calcium Element Symbols and Numbers

Easy way to memorize the Valency of first 20 Elements. YouTube
The first 20 elements in a periodic table are Hydrogen ( H - 1), Helium ( He - 2), Lithium ( Li - 3), Berelium ( Be - 4), Boron ( B - 5), Carbon ( C - 6), Nitrogen ( N - 7), Oxygen ( O - 8), Fluorine ( F - 9), Neon ( Ne - 10), Sodium ( Na - 11), Magnesium ( Mg - 12), Aluminium ( Al - 13), Silicon ( Si - 14), Phosphorus ( P - 15), Sulphur ( S - 1.

Valency Trick Trick to find valency of 51 to 60 elements,Trick to learn valency of 51 to 60
The valency of the first 30 elements of the periodic table is given below. Periodic Trends in the Oxidation States of Elements 1. Variation Of Oxidation State Along a Period While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8.

A simple way to understand and memorise the valency of first 20 elements of the periodic table
This. Valence table gives the Valence of all the elements of periodic table.Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Valence' headers to sort.

Periodic Table Of Elements With Atomic Mass And Valency
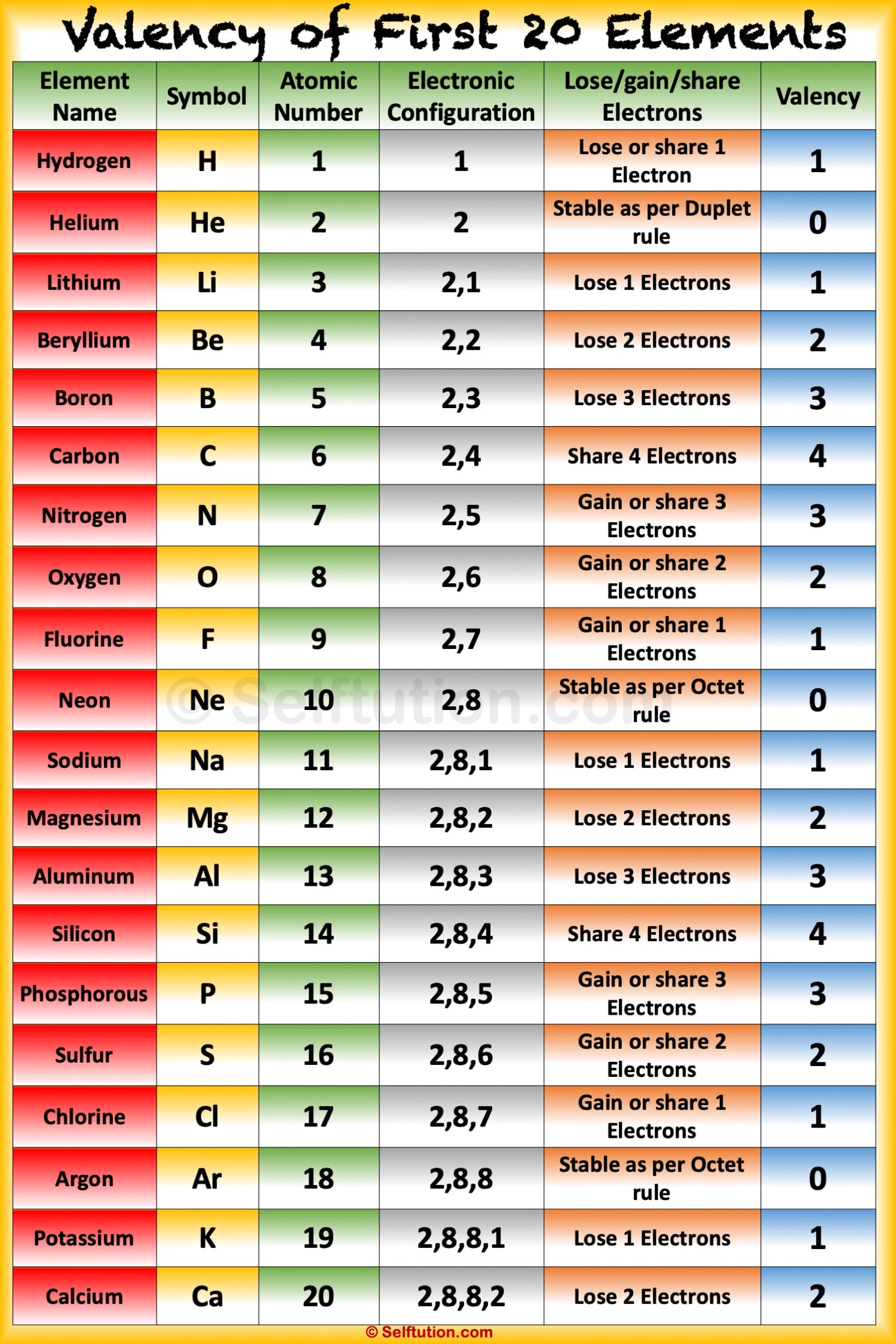
Solution Valency: "The electrons present in the outermost shell of an atom are known as the valence electrons." "Valency is the combining capacity of an atom." The valency and valence electrons for the first 20 elements are discussed below: Suggest Corrections 953 Similar questions

chemistry first 20 elements table with electronic configuration and valency Brainly.in
Valency of first 20 elements in tabular form Electronic Configuration The arrangement of electrons in the various shells of an atom of the element is called electronic configuration. The maximum electrons which can be accommodated in K shell are 2, for L shell is 8, for M shell is 18 and for N shell is 32.

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Elements Their Atomic
1.8K Share 146K views 2 years ago The Periodic Table In less than 2 minutes, you'll remember the valency of elements 1 - 30! We'll use 2 valency tricks - valency for the first 20.

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Do some elements have
This video will help students to understand electronic configuration, valence electrons and valency.For more videos on Physics, Chemistry and Mathematics ref.

Periodic Table With Names And Atomic Mass Number Valency Awesome Home
Periodic Table -. The periodic table of elements graphic is the most common tool to utilized to compute valency in this method. All of the metals in column 1 have a valency of +1, for example, hydrogen, lithium, sodium, and so on. Similarly, all of the elements in column 17 have valency 1, including fluorine, chlorine, and other elements.
Valency and Variable Valency Valence Shell and Electrons » Selftution
The valency of elements from 1 to 20 showcases some fascinating chemical behavior. Here are the valencies of selected elements: Hydrogen (H): Hydrogen typically exhibits a valency of +1, forming compounds like H2O (water) and HCl (hydrochloric acid).

periodic table of elements printable flashcards chemistry etsy find various types of valency
The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a valence of 1. Usually it's oxidation state is +1, but it can lose the electron and have a valence of -1. The most stable oxidation state is one that.

Identifying the First 20 Elements Periodic Table Activity
List of the First 20 Elements The elements are listed in order of increasing atomic number. The atomic number is the number of protons in atoms of each element. The first 20 elements and their symbols are: Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O) Fluorine (F) Neon (Ne) Sodium (Na)

What Are the First 20 Elements Names and Symbols
First 20 Elements The first 20 elements of the periodic table have been tabulated below, along with their symbols and atomic numbers. Learn more ⇒ Interactive Periodic Table Table of Contents What Information does the Atomic Number of an Element Provide? Why is Potassium denoted by the symbol 'K' and Sodium by the symbol 'Na'? Recommended Videos